Biofluorescence
Biofluorescence has always intrigued scientists. The discovery of the green fluorescent protein in Cnidaria has enabled a myriad of molecular techniques upon which much research and modern medicine is now dependent. Fluorescence occurs as specialized molecules called chromophores absorb high energy wavelength light and become excited bumping electrons to higher energy orbits (Matz et al 2002). The chromophores then relax re-emitting the light with additional energy release such that it is released at a lower wavelength (Matz et al 2002).
Fluorescing chromophores are often only active when held within a specific protein residue structure, the most famous of which is Green Fluorescent Protein (GFP) mentioned above (Shimomura 2005). GFP was first discovered in Aequoria victoria in 1961 and since then has become a crucial marker for gene expression (Shimomura 2005). GFP-like proteins have been identified spanning many wavelengths, including red fluorescent proteins (Peterson et al 2003). E. quadricolor expresses both a GFP and a unique far-red shifted RFP eqFP611 which emits light maximally at 611 nm. (Peterson et al 2003). This is the highest natural emission maximum yet recorded (Peterson et al 2003). The discovery of eqFP611 is important for multicolour microscopy and it has superior applications in tissue and full body imaging (Piatkevich et al 2010).
The presence of fluorescent proteins in E. quadricolor at Heron Island was investigated in a small study using fluorescing microscopy. In, particularly, I aimed to broadly characterize the distribution of GFP and RFP across an individual's epithelial tissue. Images were taken of both live specimens on the island and sectioned tentacles back at the University of Queensland.
On the Island
A live specimen, relaxed using MgCl, were observed under an Olympus SZx16 fluorescing dissecting microscope, whilst at Heron Island Research Station. The anemone was found to fluoresce detectably under two channels; GFP and RFP2. The excitation and emission spectrums for each channel are recorded in Table 1.
Table 1: Excitation and Emission wavelength spectrum (nm) for GFP and RFP2 filters of Olympus SZx16
|
GFP (nm)
|
RFP2 (nm)
|
|
Excitation
|
460 - 480
|
535 - 555
|
|
Emission
|
495 - 540
|
570 - 625
|
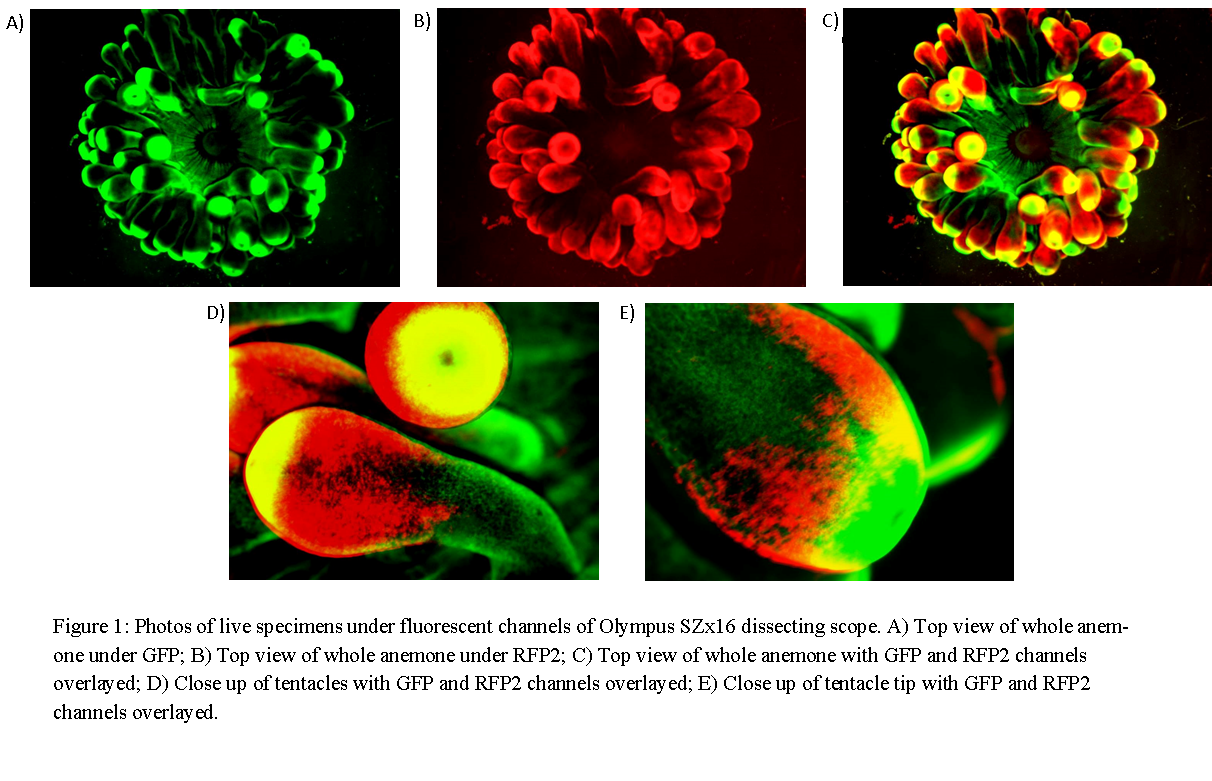
Images revealed that fluorescence was not constant across the anemone. Under GFP, tentacle tips fluoresced most strongly with limited fluorescence also observed along the tentacle trunks and oral disk (Figure 1). Fluorescence under RFP2 was concentrated at the distal end of the tentacles except for the extreme tip with little or no fluorescence on the oral disk (Figure 1).
To investigate the distribution of GFP and RFP more closely a single tentacle was removed from this specimen and prepared for sectioning back at the university of Queensland. Whilst on Heron Island the anemone was relaxed with MgCl and the single tentacle was removed and fixed in 4% paraformaldehyde diluted in saltwater and left for 24 hours. The tentacle was then stepped into ethanol for preservation and transport to the University of Queensland.
At University of Queensland
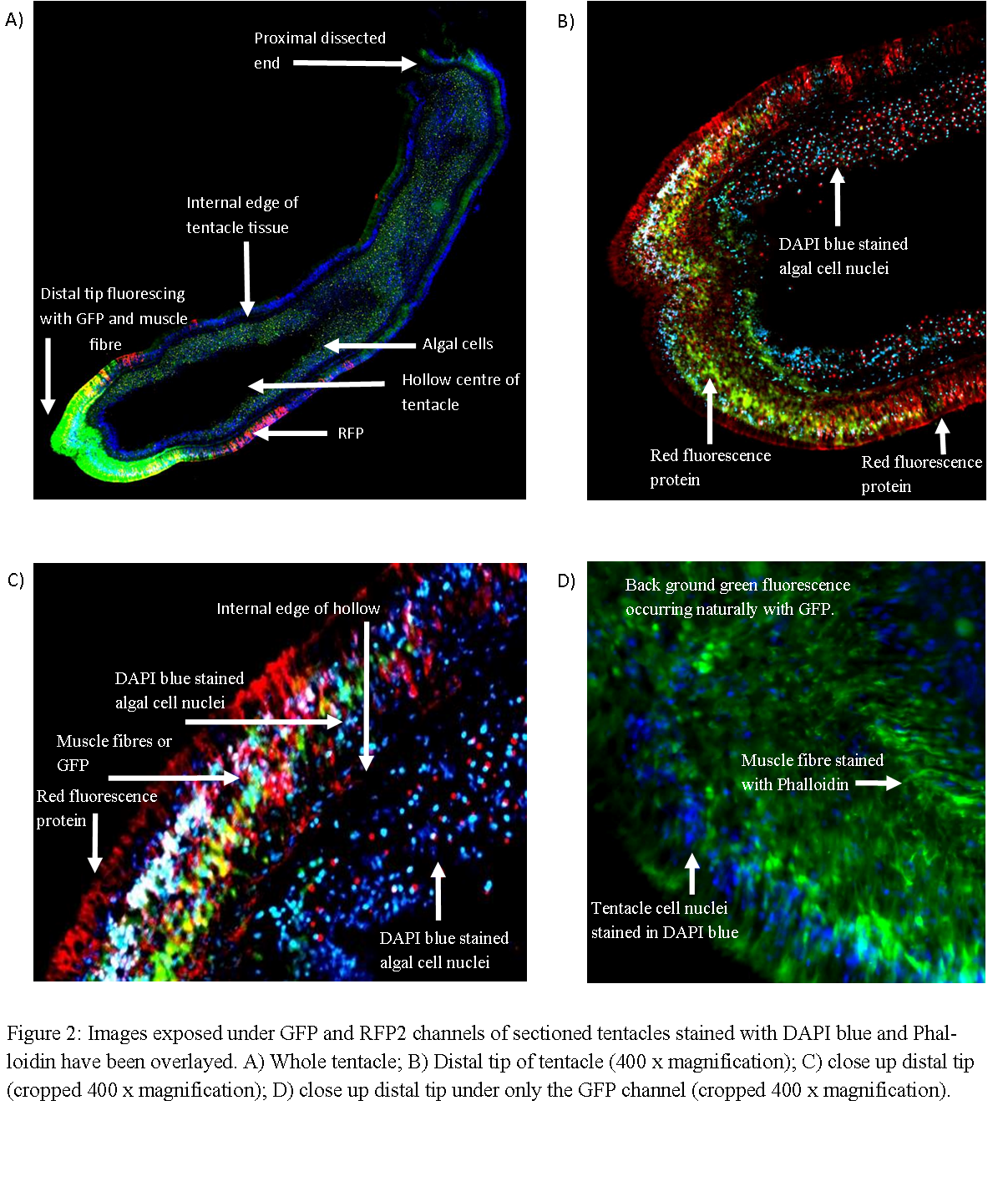
At the university the tentacle was sectioned across a sagittal cross-section and mounted on slides. The slides were stained with DAPI (blue) and phalloidin. DAPI (blue) fluoresces blue at 461 nm and binds to A-T rich regions highlighting cell nuclei and Phalloidin fluorescing green at 600 nm, binding to actin filaments and therefore highlighting muscle fibers. Cover slips were attached using pro-line gold and slides were refrigerated before examination under a Nikon Inverted Eclipse Ti fluorescing compound scope.
The sectioned tentacles revealed similar patterns of fluorescence as live specimens. Figure 2A shows GFP fluorescing most strongly at the tentacle tip whilst RFP fluorescing at the distal end but back from the extreme tip. Reducing the exposure of the the green channel during post processing revealed that RFP is present at the tentacle tip, however perhaps at lower quantities such that maybe ordinarily masked by GFP (Figure 2B&C). The application of DAPI blue stain clearly reveals the hollow nature of the tentacle with the internal surface of tentacle tissue visible (Figures 2A,B and C). Staining with Phalloidin, which fluoresces, green reveals circular muscular filaments (Figure 2D). However, staining with this dye makes it difficult to discern the true expression of GFP given both fluoresce green. However, the distal tip is thought contain high concentration of GFP not stain as the level of fluorescence at the tip is well beyond that else where thought to represent muscle fibers (Figure 2A).
This work has been able to characterize broad patterns of GFP and RFP distribution across E. quadricolor epithelial tissues. The importance of florescence to anemone ecology is unknown and as such the importance of distributive patterns of GFP and RFP is also unknown. One, uninvestigated theory is that fluorescence is associated with the anemones carnivorous behaviour. It is common knowledge that luminescence has long been associated with prey capture in deep sea angler fish. However, it has recently been shown that carnivorous prey trapping plants have blue fluorescent emission at capture spots in their traps (Kurup et al 2013). It was found that masking such emission darastically reduced prey capture success (Kurup et al 2013). It is suggested here that a similar system my be evoked in E. quadricolor such that the fluorescent patterns may be used to attract prey towards sedentary anemone. Further work is needed to investigate this theory.
|